An NBD-based two-in-one Cu2+/Ni2+ chemosensor with differential charge transfer processes†
Abstract
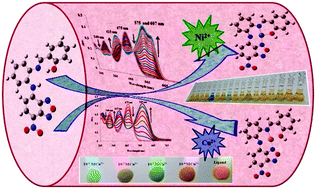
A new multifunctional NBD-based probe has been developed having high sensitivity and selectivity towards Cu2+/Ni2+ over other metal ions over a wide pH range. The probe 1 could be used for the simultaneous estimation of Cu2+ and Ni2+ ions through its visible color change from yellow to blue and light green, respectively, in semi-aqueous systems. The binding mode of probe 1 to Cu2+ and Ni2+ was determined to be a 1 : 1 complexation stoichiometry using a Job's plot. Ethylenediamine tetraacetate showed high selectivity towards the in situ prepared Cu2+ complex over the Ni2+ complex, which is applied to distinguish Ni2+ and Cu2+ ions. The detection limits for Cu2+ and Ni2+ ions, 1.8 × 10−7 M and 1.1 × 10−6 M, respectively, were lower than those recommended by the WHO guidelines for drinking water. Moreover, probe 1 could be used as a practical, visible colorimetric test kit for Cu2+ ions. The sensing mechanism of Cu2+ and Ni2+ ions was supported by DFT-calculations.

 Please wait while we load your content...
Please wait while we load your content...