Synthesis of enantiomerically pure bis(2,2-dimethyl-1,3-dioxolanylmethyl)chalcogenides and dichalcogenides†
Abstract
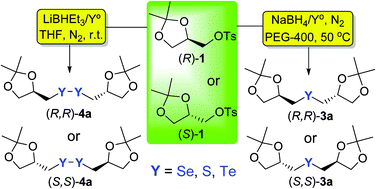
Herein we describe the reaction between the nucleophilic species of chalcogenium (sulfur, selenium and tellurium) and (R)- and (S)-solketal tosylates to prepare enantiomerically pure bis-1,3-dioxolanylmethyl chalcogenides and dichalcogenides in short reaction times and good yields. Furthermore, the new diselenide 4a was evaluated in the Michael reaction with acrylonitrile, giving (R)-3-[(2,3-dihydroxypropyl)selanyl]propanenitrile 6b after two steps.

 Please wait while we load your content...
Please wait while we load your content...