Design and microwave assisted synthesis of novel 2-phenyl/2-phenylethynyl-3-aroyl thiophenes as potent antiproliferative agents†‡
Abstract
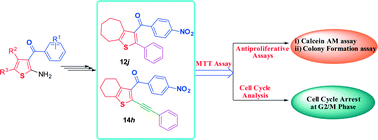
In the present study, 2-phenyl/2-phenylethynyl-3-aroyl thiophenes have been designed and synthesized via microwave assisted methods. All the synthesized compounds were evaluated for in vitro antiproliferative activity against various human cancer cell lines. Compounds 12j and 14h were found to be the most promising compounds against all the tested cancer cell lines, particularly against A-375 (IC50 = 1.07 ± 0.1 and 0.81 ± 0.1 μM, respectively) and MIA PaCa-2 (IC50 = 5.35 ± 0.6 and 3.00 ± 1.0 μM, respectively) cancer cell lines, which are comparable to the standard paclitaxel. Further, the antiproliferative activity of the most potent compounds 12j and 14h was confirmed by calcein AM and clonogenic assays and was found to induce cell cycle arrest at the G2/M phase, suggesting that cell exposure to selected derivatives produces mitotic failure. In silico ADME studies confer the oral drug like characteristics of the potent compounds.

 Please wait while we load your content...
Please wait while we load your content...