Abstract
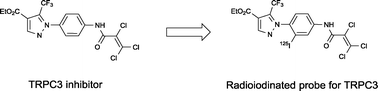
The transient receptor potential canonical 3 (TRPC3) channel is a member of the TRPC family that contributes to the entry of Ca2+ through the plasma membrane or modulates the driving force for Ca2+ entry channels. The pyrazole compound Pyr3 has recently been reported to be a selective TRPC3 inhibitor and has become an attractive research tool and therapeutic agent for the treatment of heart failure. However, the in vivo characteristics of Pyr3 have not been investigated. To monitor the fate of Pyr3 in vivo, we designed and synthesized a radioiodinated Pyr3 probe ([125I]I-Pyr3) by introducing radioiodine at the 2-position of the central phenyl ring of Pyr3. I-Pyr3 was shown to have direct TRPC3 inhibition activity similar to that of Pyr3 in TRPC3-overexpressing HEK293 cells. Using the tributyltin derivative as a radioiodination precursor, [125I]I-Pyr3 was successfully prepared with high radiochemical purity. Biodistribution studies of [125I]I-Pyr3 and [125I]I-Pyr8 (the esterolysis product of [125I]I-Pyr3) indicated high uptake of intact [125I]I-Pyr3 in the lung and rapid metabolism to [125I]I-Pyr8. These findings provide useful information about the in vivo kinetics of the selective TRPC inhibitor Pyr3.

 Please wait while we load your content...
Please wait while we load your content...