N-Butylpyrrolidinone as a dipolar aprotic solvent for organic synthesis†
Abstract
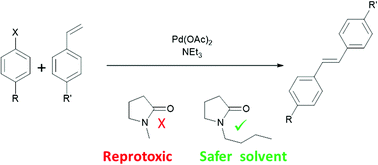
Dipolar aprotic solvents such as N-methylpyrrolidinone (or 1-methyl-2-pyrrolidone (NMP)) are under increasing pressure from environmental regulation. NMP is a known reproductive toxin and has been placed on the EU “Substances of Very High Concern” list. Accordingly there is an urgent need for non-toxic alternatives to the dipolar aprotic solvents. N-Butylpyrrolidinone, although structurally similar to NMP, is not mutagenic or reprotoxic, yet retains many of the characteristics of a dipolar aprotic solvent. This work introduces N-butylpyrrolidinone as a new solvent for cross-coupling reactions and other syntheses typically requiring a conventional dipolar aprotic solvent.

 Please wait while we load your content...
Please wait while we load your content...