Safety evaluation and antihyperlipidemia effect of aqueous extracts from fermented puerh tea
Abstract
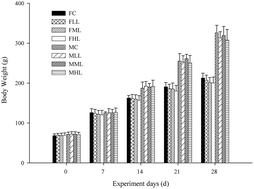
Fermented puerh tea, having undergone a long period of secondary oxidization and fermentation, has become more and more popular in recent years. In the present paper, a safety evaluation of aqueous extracts from fermented puerh tea (EFPT) was performed, including an oral acute toxicity study in rats and mice, mutation tests, a mouse micronucleus test, mouse sperm abnormality test and a 30 day feeding study in rats. Meanwhile, the antihyperlipidemia effect of EFPT was investigated as well. It was found that the oral maximum tolerated dose of EFPT was more than 10.0 g per kg body weight both in rats and mice. And it had no mutagenicity as judged by negative experimental results of the mutation test. No abnormal symptoms, clinical signs or deaths have been found in rats in each group throughout the experiments. In addition, EFPT in this study showed certain effects on hyperlipidemia.

 Please wait while we load your content...
Please wait while we load your content...