High efficiency hydrogen evolution from native biomass electrolysis†
Abstract
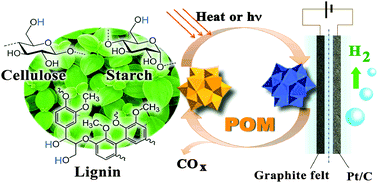
A novel electrolysis approach for hydrogen evolution directly from native biomasses, such as cellulose, lignin and even wood and grass powders, to hydrogen at low temperature is presented. Using aqueous polyoxometalate (POM) as a catalyst at the anode, the raw biomass is oxidized and electrons are transferred to POM molecules by heating or light-irradiation. Protons from biomass diffuse to the cathode and are reduced to hydrogen. The electric energy consumption could be as low as 0.69 kW h per normal cubic meter of H2 (Nm−3 H2) at 0.2 A cm−2, which is only 16.7% of the energy consumed for the reported water electrolysis. Unlike the traditional electrolysis of alcohols, a noble-metal catalyst is not required at the anode.

 Please wait while we load your content...
Please wait while we load your content...