Kagome-type isostructural 3D-transition metal fluorosulfates with spin 3/2 and 1: synthesis, structure and characterization†
Abstract
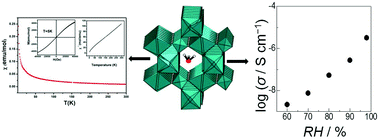
Two isostructural transition metal fluorosulfates based on Co and Ni metal ions with the molecular composition of [H3O][M(SO4)F] (where M = Co(II) for 1 and Ni(II) for 2) were synthesized under solvothermal conditions and structurally characterized by single crystal X-ray analysis. The materials were further characterized by complementary techniques like TGA, FTIR and PXRD. The 3D-crystal lattice consists of a kagome-type entity where sulfate groups replaced one of the metal nodes when compared with true kagome structures. Magnetic studies of the complexes were also performed which showed that the interactions at the metal center are antiferromagnetic in nature. The proton conductivity increases with the increase in humidity and was found to be 7.9 × 10−6 S cm−1 for 2 at RH = 98%.

 Please wait while we load your content...
Please wait while we load your content...