Electrophilic iodination: a gateway to high iodine compounds and energetic materials†
Abstract
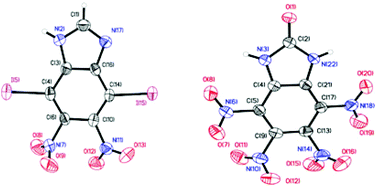
A large number of iodine atoms can be introduced into a single molecule in a one-pot reaction using trifluoroperacetic acid-mediated electrophilic iodination methodology. The scope of this reaction was investigated extensively using several pyrazole substrates which resulted in nine polyiodo pyrazole compounds with iodine content as high as 80%. This synthetic methodology was also utilized successfully for iodination of benzimidazoles. Tetraiodobenzimidazole was nitrated with 100% nitric acid to give a high yield of 4,5,6,7-tetranitro-1H-benzimidazol-2(3H)-one (14). All of these materials were fully characterized and compounds 5, 9, 10 and 14 were confirmed further with single crystal X-ray analysis. High density, positive oxygen balance, and very good impact sensitivity values characterize 14. For the first time, two 1,2,5-oxadiazole-N-oxide rings were introduced into a benzimidazole ring (11) which remarkably improves the stability of oxadiazole-N-oxide compounds.

 Please wait while we load your content...
Please wait while we load your content...