Different magnetic responses observed in Co II4, Co II3 and Co II1-based MOFs†
Abstract
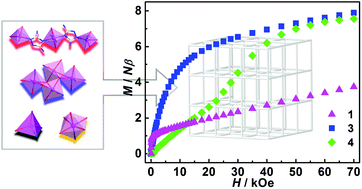
Four magnetic MOFs with anisotropic CoII ions, {[Co5(H2O)2(μ3-OH)2(atz)2(stp)2]·1.5H2O}n (1), {[Co5(H2O)2(μ3-OH)2(trz)2(stp)2]·1.3H2O}n (2), {[Co5(H2O)6(μ3-OH)2(trz)2(stp)2]·2.5CH3OH}n (3) and {[Co3(H2O)4(Hdatrz)2(stp)2]·3H2O}n (4) (stp3− = 2-sulfoterephthalate, trz− = 1,2,4-triazolate, atz− = 3-amino-1,2,4-triazolate and Hdatrz = 3,5-diamino-1,2,4-triazole) were solvothermally isolated by varying the substituent groups appended on the N-heterocyclic triazole and structurally and magnetically characterized. Structural analyses indicate that the former two complexes are crystallographically isostructural, exhibiting pillared-layer frameworks with mixed triazolyl and carboxylate extended CoII4 + CoII1 layers supported by rigid stp3− connectors. Complex 3 is built from butterfly-shaped CoII4 cluster-based layers, which are interconnected with single cobalt(II) octahedra by ditopic stp3− bridges. By contrast, complex 4 consists of linear {Co3(μ-N1,N4-Hdatrz)2} subunits, which are extended by 3-connected stp3− linkers into a stable 3D framework. Magnetically, 1 exhibits ferromagnetic ordering below 2.7 K due to the well-organized alignment of the non-compensated resultant moment from octahedra and tetrahedral cobalt(II) carriers, while 3 is in a non-zero paramagnetic state above 2.0 K resulting from the coexistence of intermetallic ferromagnetic and antiferromagnetic interactions. The magnetic competition between weak inter-subunit antiferromagnetic interactions and the external magnetic field makes 4 behave as a field-induced metamagnet with a critical field of 27.5 kOe. These interesting magnetostructural results suggest that the anisotropy of the moment carrier and the interlayer/intersubunit separations significantly dominate the magnetic responses in extended MOFs, providing an informative platform for the further development of interesting magnetic materials, both of academic and industrial interest.

 Please wait while we load your content...
Please wait while we load your content...