The chemistry of parent phosphiranide in the coordination sphere of tungsten†
Abstract
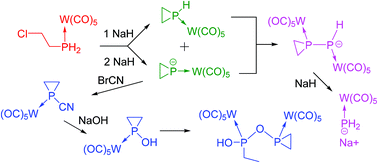
2-Chloroethylphosphine W(CO)5 complex 1 readily reacts with sodium hydride. With one equivalent of NaH, the parent phosphirane complex 3 is obtained. With more than two equivalents, the phosphiranide complex 2 is exclusively formed. With 1.5 equivalents, a 1 : 1 mixture of 2 and 3 is obtained but 2 readily attacks 3 at the phosphorus atom by splitting of ethylene and by the formation of the P–P complex 4. In turn, the P–P bond of 4 is split by NaH to yield the phosphide complex 8. The phosphiranide complex 2 is a good source for a large variety of functional phosphirane complexes 9. With BrCN, the 1-cyanophosphirane complex 9a is formed. Upon heating it loses its complexing group. Upon hydrolysis, it gives the 1-hydroxyphosphirane complex 12 which dimerizes in basic medium by opening one P–C bond of the ring to give 13. The reaction of 2 with PhPCl2 yields the triphosphorus complex 9g whose molecular structure has been established by X-ray crystal structure analysis.

 Please wait while we load your content...
Please wait while we load your content...