C3-symmetry Mo3S4 aminophosphino clusters combining three sources of stereogenicity: stereocontrol directed by hydrogen bond interactions and ligand configuration†
Abstract
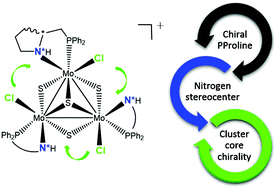
A diastereoselective synthesis of proline containing aminophosphino cubane-type Mo3S4 clusters, (P)-[Mo3S4Cl3((1S,2R)-PPro)3]Cl ((P)-[Mo-(SN,RC)]Cl) and (P)-[Mo3S4Cl3((1S,2S)-PPro)3]Cl ((P)-[Mo-(SN,SC)]Cl), has been achieved in high yields by reacting the corresponding enantiomerically pure PPro ((R)- and (S)-2-[(diphenylphosphino)methyl]pyrrolidine) ligands with the Mo3S4Cl4(PPh3)3(H2O)2 complex. Circular dichroism, nuclear magnetic resonance and X-ray techniques confirm that the (P)-[Mo-(SN,RC)]Cl and (P)-[Mo-(SN,SC)]Cl cluster cations are diastereoisomers which combine three sources of stereogenicity provided by the cluster framework, one carbon atom of the aminophosphine ligand and the nitrogen stereogenic center. The higher stability of the (P)-[Mo-(SN,SC)]+ cation is due to stabilizing vicinal Cl⋯HN interactions as well as due to the cis-fused conformation of the bicyclic system formed upon coordination of the aminophosphine ligand.


 Please wait while we load your content...
Please wait while we load your content...