Emission behaviour of a series of bimetallic Cd(ii)–Au(i) coordination polymers†
Abstract
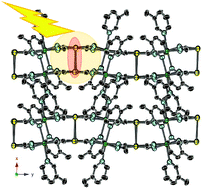
A series of bimetallic coordination polymers with the elemental composition of [CdIIL2][Au(CN)2]2, (L = 3-methylpyridine, 4-ethylpyridine, 3,5-lutidine and 3-fluoropyridine) were synthesized and their crystal structures were determined. In all of the investigated compounds, there existed a pair of Au–Au atoms whose interatomic distance was shorter than the sum of van der Waals radii (0.36 nm) as an indication of the aurophilic interaction. The compounds were emissive under the irradiation at 370 nm. The emission spectra recorded in the temperature range of 183–363 K were characterized by the vibronic structures with a peak spacing (Δν) of ca. 2000 cm−1. The value of Δν was close to the stretching vibration of the coordinated C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (2150–2170 cm−1). It was postulated that C
N (2150–2170 cm−1). It was postulated that C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups participated in the emission processes through their vibronic coupling. In the case of L = 4-ethylpyridine, the lifetime of emission was measured in the same temperature range, leading to the conclusion that the activation energy of the non-radiative processes (ΔEa) was estimated to be 20 kJ mol−1.
N groups participated in the emission processes through their vibronic coupling. In the case of L = 4-ethylpyridine, the lifetime of emission was measured in the same temperature range, leading to the conclusion that the activation energy of the non-radiative processes (ΔEa) was estimated to be 20 kJ mol−1.

 Please wait while we load your content...
Please wait while we load your content...