Mechanism of iridium-catalysed branched-selective hydroarylation of vinyl ethers: a computational study†
Abstract
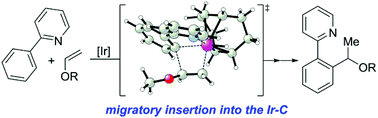
The iridium-catalysed branched-selective hydroarylation of vinyl ethers represents a rare example of the branched-selective hydroarylation involving the non-styrene-type alkenes. Herein, we report our DFT calculations on the mechanism of this reaction. The results show that after C–H oxidative addition, instead of the widely accepted Chalk–Harrod type mechanism, the branched-selective hydroarylation may proceed through an unconventional modified Chalk–Harrod type mechanism, involving the migratory insertion into the Ir–C bond and C–H reductive elimination. Both steric and electronic effects of the alkoxy group were found to account for the complete branched selectivity.

 Please wait while we load your content...
Please wait while we load your content...