Fluorescent asymmetric bis-ureas for pyrophosphate recognition in pure water†
Abstract
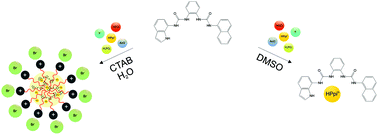
Three fluorescent asymmetric bis-urea receptors (L1–L3) have been synthesised. The binding properties of L1–L3 towards different anions (fluoride, acetate, hydrogencarbonate, dihydrogen phosphate, and hydrogen pyrophosphate HPpi3−) have been studied by means of 1H-NMR, UV-Vis and fluorescence spectroscopy, single crystal X-ray diffraction, and theoretical calculations. In particular, a remarkable affinity for HPpi3− has been observed in the case L1 (DMSO-d6/0.5% H2O) which also acts as a fluorimetric chemosensor for this anion. Interestingly, when L1 is included in cetyltrimethylammonium (CTAB) micelles, hydrogen pyrophosphate recognition can also be achieved in pure water.


 Please wait while we load your content...
Please wait while we load your content...