Red emission generation through highly efficient energy transfer from Ce3+ to Mn2+ in CaO for warm white LEDs
Abstract
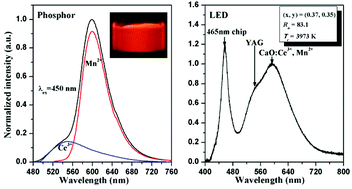
CaO:Ce3+,Mn2+ phosphors with various Mn2+ concentrations were synthesized by a solid state reaction method. Efficient energy transfer from Ce3+ to Mn2+ was observed and it allows the emission color of CaO:Ce3+,Mn2+ to be continuously tuned from yellow (contributed by Ce3+) to red (by Mn2+) with an increase in Mn2+ concentration and upon blue light excitation. The red emission becomes dominant when the Mn2+ concentration is ≥0.014 with an energy transfer efficiency higher than 87% which can reach as high as 94% for a Mn2+ concentration of only 0.02. A critical distance of 10.5 Å for the Ce3+–Mn2+ energy transfer was determined. A faster decrease of Ce3+ luminescence intensity in comparison with its lifetime was observed on increasing the Mn2+ concentration. The analysis of this feature reveals that the Ce3+ excitation energy can be completely transferred to Mn2+ if the Ce3+–Mn2+ distance is shorter than 7.6 Å. A warm white LED was fabricated through integrating an InGaN blue LED chip and a blend of two phosphors (YAG:Ce3+ yellow phosphor and CaO:0.007Ce3+,0.014Mn2+ red phosphor) into a single package, which has CIE chromaticity coordinates of (x = 0.37, y = 0.35), a correlated color temperature of 3973 K and a color rendering index of 83.1. The results indicate that CaO:Ce3+,Mn2+ may serve as a potential red phosphor for blue LED based warm white LEDs.


 Please wait while we load your content...
Please wait while we load your content...