Fe/γ-Al2O3 and Fe–K/γ-Al2O3 as reverse water-gas shift catalysts†
Abstract
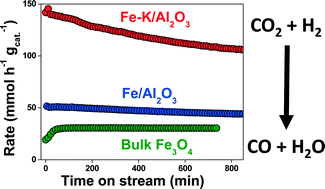
The reverse water-gas shift (RWGS) reaction was investigated on Fe/γ-Al2O3 and Fe–K/γ-Al2O3 catalysts at temperatures between 723 K and 753 K and atmospheric pressure. Both materials exhibited fast catalytic CO formation rates and high CO selectivity (>99%). Reaction rates displayed a strong dependence on H2 partial pressure (reaction orders of 0.58 and 0.54 on Fe/γ-Al2O3 and Fe–K/γ-Al2O3, respectively), and a weak dependence on CO2 partial pressure (reaction orders of 0.37 and 0.21, respectively) under nearly equimolar CO2 : H2 composition. The catalysts were stable under excess H2 but deactivated slowly (1–2% h−1 of the overall reaction rate) under an equimolar mixture of CO2 and H2. Addition of potassium to the Fe/γ-Al2O3 material (Fe/K mass ratio = 1.24) led to a threefold increase in reaction rate, but also doubled the deactivation rate (CO2 : H2 = 1 : 1). Gas-switching experiments (CO2 or H2 only) and DRIFTS spectra collected in situ showed that stable intermediates formed on Fe–K/Al2O3 but not on Fe/Al2O3. This suggests, although does not prove, that a redox mechanism is the only reaction pathway on the Fe/Al2O3 catalyst, and is the predominant pathway on the Fe–K/Al2O3 catalyst. The potassium promoter activates a secondary pathway for CO formation, which may be the so-called associative pathway.

 Please wait while we load your content...
Please wait while we load your content...