Titanate nanotube-promoted chemical fixation of carbon dioxide to cyclic carbonate: a combined experimental and computational study†
Abstract
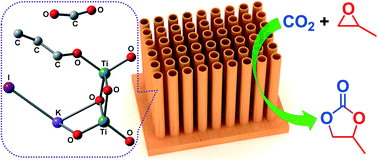
Titanate nanotubes (TNT) were found to be air- and water-tolerant, efficient, and recyclable Lewis acid catalysts towards the cycloaddition of carbon dioxide (CO2) to propylene oxide (PO) for the synthesis of cyclic propylene carbonate (PC). Using TNT as the catalyst and potassium iodide, tetrabutylammonium bromide or tetraphenylphosphonium bromide as the co-catalyst, PO was readily converted to PC with a yield of up to >99.9% and a PC selectivity of 100% under relatively mild conditions. Experimental research revealed that the TNT catalyst with the highest exposure of active sites, including surface hydroxyl groups and Lewis acid sites, proved to be the most active for the cycloaddition reaction. Our theoretical evaluation based on density functional theory calculations further indicated that the high Lewis acidity of TNT strongly reduces the energy barrier for the ring-opening step through the polarization of the C–O bond of PO, which allows a subsequent nucleophilic attack easily. Both experimental and computational studies demonstrated that the synergetic effect between TNT and the co-catalyst plays an important role in PC formation. The TNT catalyst is richer in surface Lewis acid sites, shows a large surface area and mesoporous structure, and has a heterogeneous and recyclable nature, which makes TNT an excellent, interesting and ideal catalyst for chemical fixation of CO2.

 Please wait while we load your content...
Please wait while we load your content...