Dielectric characteristics of fast Li ion conducting garnet-type Li5+2xLa3Nb2−xYxO12 (x = 0.25, 0.5 and 0.75)
Abstract
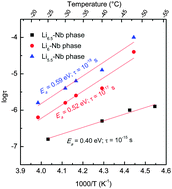
Here, we report the dielectric characteristics of Li-stuffed garnet-type Li5+2xLa3Nb2−xYxO12 (x = 0.25, 0.5 and 0.75) in the temperature range about −53 to 50 °C using AC impedance spectroscopy. All the investigated Li-stuffed garnet compounds were prepared, under the same condition, using conventional solid-state reaction at elevated temperature in air. The Nyquist plots show mainly bulk contribution to the total Li+ ion conductivity for Li5.5La3Nb1.75Y0.25O12 (Li5.5–Nb) and Li6La3Nb1.5Y0.5O12 (Li6–Nb), while both bulk and grain-boundary effects are visible in the case of Li6.5La3Nb1.25Y0.75O12 (Li6.5–Nb) phase at ∼−22 °C. Non-Debye relaxation process was observed in the modulus AC impedance plots. The dielectric loss tangent of Li5+2xLa3Nb2−xYxO12 are compared with that of the corresponding Ta analogue, Li5+2xLa3Ta2−xYxO12 and showed a decrease in peak intensity for the Nb-based garnet samples which may be attributed to a slight increase in their Li+ ion conductivity. The relative dielectric constant values were also found to be higher for the Ta member (>60 for Li5+2xLa3Ta2−xYxO12) than that of the corresponding Nb analogue (∼50 for Li5+2xLa3Nb2−xYxO12) at below room temperature. A long-range order Li+ ion migration pathway with relaxation time (τ0) 10−18–10−15 s and an activation energy of 0.59–0.40 eV was observed for the investigated Li5+2xLa3Nb2−xYxO12 garnets and is comparable to that of the corresponding Ta-based Li5+2xLa3Ta2−xYxO12 garnets.

 Please wait while we load your content...
Please wait while we load your content...