Photo-induced oxidation of the uniquely liganded heme f in the cytochrome b6f complex of oxygenic photosynthesis†
Abstract
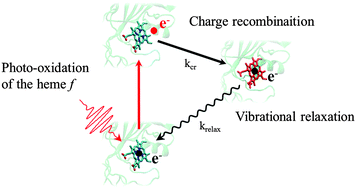
The ultrafast behavior of the ferrous heme f from the cytochrome b6f complex of oxygenic photosynthesis is revealed by means of transient absorption spectroscopy. Benefiting from the use of microfluidic technologies for handling the sample as well as from a complementary frame-by-frame analysis of the heme dynamics, the different relaxation mechanisms from vibrationally excited states are disentangled and monitored via the shifts of the heme α-absorption band. Under 520 nm laser excitation, about 85% of the heme f undergoes pulse-limited photo-oxidation (<100 fs), with the electron acceptor being most probably one of the adjacent aromatic amino acid residues. After charge recombination in 5.3 ps, the residual excess energy is dissipated in 3.6 ps. In a parallel pathway, the remaining 15% of the hemes directly relax from their excited state in 2.5 ps. In contrast to a vast variety of heme-proteins, including the homologous heme c1 from the cytochrome bc1 complex, there is no evidence that heme f photo-dissociates from its axial ligands. Due to its unique binding, with histidine and an unusual tyrosine as axial ligands, the heme f exemplifies a dependence of ultrafast dynamics on the structural environment.

 Please wait while we load your content...
Please wait while we load your content...