Dynamics of human acetylcholinesterase bound to non-covalent and covalent inhibitors shedding light on changes to the water network structure†
Abstract
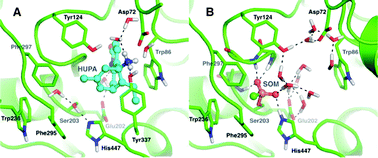
We investigated the effects of non-covalent reversible and covalent irreversible inhibitors on human acetylcholinesterase and human butyrylcholinesterase. Remarkably a non-covalent inhibitor, Huperzine A, has almost no effect on the molecular dynamics of the protein, whereas the covalently binding nerve agent soman renders the molecular structure stiffer in its aged form. The modified movements were studied by incoherent neutron scattering on different time scales and they indicate a stabilization and stiffening of aged human acetylcholinesterase. It is not straightforward to understand the forces leading to this strong effect. In addition to the specific interactions of the adduct within the protein, some indications point towards an extensive water structure change for the aged conjugate as water Bragg peaks appeared at cryogenic temperature despite an identical initial hydration state for all samples. Such a change associated to an apparent increase in free water volume upon aging suggests higher ordering of the hydration shell that leads to the stiffening of protein. Thus, several additive contributions seem responsible for the improved flexibility or stiffening effect of the inhibitors rather than a single interaction.

 Please wait while we load your content...
Please wait while we load your content...