Evaluating the solid electrolyte interphase formed on silicon electrodes: a comparison of ex situ X-ray photoelectron spectroscopy and in situ neutron reflectometry†
Abstract
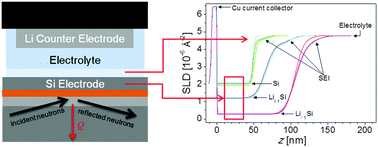
This work details the in situ characterization of the interface between a silicon electrode and an electrolyte using a linear fluorinated solvent molecule, 0.1 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in deuterated dimethyl perfluoroglutarate (d6-PF5M2) (1.87 × 10−2 mS cm−1). The solid electrolyte interphase (SEI) composition and thickness determined via in situ neutron reflectometry (NR) and ex situ X-ray photoelectron spectroscopy (XPS) were compared. The data show that SEI expansion and contraction (breathing) during electrochemical cycling were observed via both techniques; however, ex situ XPS suggests that the SEI thickness increases during Si lithiation and decreases during delithiation, while in situ NR suggests the opposite. The most likely cause of this discrepancy is the selective removal of SEI components (top 20 nm of the SEI) during the electrode rinse process, which is required to remove the electrolyte residue prior to ex situ analysis, demonstrating the necessity of performing SEI characterization in situ.


 Please wait while we load your content...
Please wait while we load your content...