Two-dimensional interlocked pentagonal bilayer ice: how do water molecules form a hydrogen bonding network?
Abstract
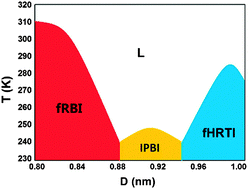
The plethora of ice structures observed both in bulk and under nanoscale confinement reflects the extraordinary ability of water molecules to form diverse forms of hydrogen bonding networks. An ideal hydrogen bonding network of water should satisfy three requirements: (1) four hydrogen bonds connected with every water molecule, (2) nearly linear hydrogen bonds, and (3) tetrahedral configuration for the four hydrogen bonds around an O atom. However, under nanoscale confinement, some of the three requirements have to be unmet, and the selection of the specific requirement(s) leads to different types of hydrogen bonding structures. According to molecular dynamics (MD) simulations for water confined between two smooth hydrophobic walls, we obtain a phase diagram of three two-dimensional (2D) crystalline structures and a bilayer liquid. A new 2D bilayer ice is found and named the interlocked pentagonal bilayer ice (IPBI), because its side view comprises interlocked pentagonal channels. The basic motif in the top view of IPBI is a large hexagon composed of four small pentagons, resembling the top view of a previously reported “coffin” bilayer ice [Johnston, et al., J. Chem. Phys., 2010, 133, 154516]. First-principles optimizations suggest that both bilayer ices are stable. However, there are fundamental differences between the two bilayer structures due to the difference in the selection among the three requirements. The IPBI sacrifices the linearity of hydrogen bonds to retain locally tetrahedral configurations of the hydrogen bonds, whereas the coffin structure does the opposite. The tradeoff between the conditions of an ideal hydrogen bonding network can serve as a generic guidance to understand the rich phase behaviors of nanoconfined water.

 Please wait while we load your content...
Please wait while we load your content...