Solvent effects on electrosynthesis, morphological and electrochromic properties of a nitrogen analog of PEDOT†
Abstract
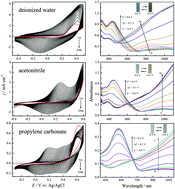
A new nitrogen analog of 3,4-ethylenedioxythiophene (EDOT), N-methyl-3,4-dihydrothieno[3,4-b][1,4]oxazine (MDTO), was electropolymerized in different solvents (deionized water, acetonitrile, and propylene carbonate) using LiClO4 as the electrolyte. The structure and performance of as-prepared PMDTO polymers were systematically studied by cyclic voltammetry, UV-vis spectroscopy, FT-IR, SEM, thermogravimetry, spectroelectrochemistry and electrochromic techniques. To our surprise, solvents had a major influence on the electropolymerization of MDTO and properties of the resultant polymers, including morphology, electrochemistry, electronic and optical properties, and electrochromics, etc. In aqueous solution, MDTO revealed the lowest onset oxidation potential (0.19 V) than in acetonitrile (0.48 V) and propylene carbonate (0.49 V). However, PMDTO films showed rather poor cycling stability in water, while outstanding stability in acetonitrile and propylene carbonate. Films prepared in propylene carbonate displayed a rather smooth morphology, lower band gap (1.65 eV), higher transparency (97.3%) and a contrast ratio (44.6%) at λ = 466 nm. PMDTO films obtained in acetonitrile showed significantly higher coloration efficiency (169.5 cm2 C−1) than in other two solvents (∼97.6 cm2 C−1) with a moderate contrast ratio (24.5%).

 Please wait while we load your content...
Please wait while we load your content...