A study on the influence of sodium carbonate concentration on the synthesis of high Mg calcites†
Abstract
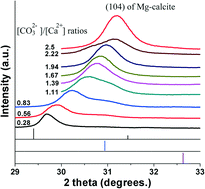
Well crystallized high Mg calcites in pure phase with Mg contents higher than 50 mol%, so-called protodolomite, have not been synthesized in the laboratories to our knowledge. In this work, the synthesis of high Mg calcites in pure phase with Mg contents controlled from 10 to 63 mol% was realized by using amorphous calcium magnesium carbonate as an intermediate precursor phase through a hydrothermal process in the absence of any organic additive. Besides the molar ratios of [Mg2+]/[Ca2+], the molar ratios of carbonate and calcium ions in the mother solutions are also very important for the Mg contents in high Mg calcites in pure phase. The higher molar ratios of [CO32−]/[Ca2+] in the mother solutions, the higher Mg contents in Mg calcites can be reached at relatively low carbonate concentrations. This is the first time that the variation of Mg contents in high Mg calcites by changing the molar ratios of [CO32−]/[Ca2+] in the mother solutions has been reported. Further increase of carbonate concentration results in the formation of other crystal phases such as Ca-magnesite, brucite, and aragonite. Reports on the structural analysis and formation mechanisms of thermodynamically unstable biogenic high-Mg calcite minerals may shed light on the preparation of functional materials with enhanced mechanical properties.

 Please wait while we load your content...
Please wait while we load your content...