Syntheses, crystal structures and photocatalytic properties of four hybrid iodoargentates with zero- and two-dimensional structures†
Abstract
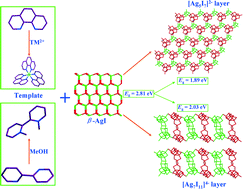
With a [TM(phen)3]2+ (TM = transition metal, phen = 1,10-phenanthroline) complex and N-alkylated [(Me)2-2,2′-bipy]2+ (Me = methyl, 2,2′-bipy = 2,2′-bipyridine) as cations, a series of hybrid iodoargentates, namely [TM(phen)3]2Ag3I7 (TM = Mn (1), Fe (2)), [Mn(phen)3]Ag5I7 (3) and [(Me)2-2,2′-bipy]2Ag7I11 (4), have been solvothermally prepared and structurally characterized. Compounds 1 and 2 feature isolated windmill-shaped [Ag3I7]4− trimers based on [AgI3] triangles, whereas compound 3 contains two-dimensional (2D) microporous [Ag5I7]2− layers composed of [Ag3I7] and [Ag7I13] secondary building units (SBUs) based on [AgI4] tetrahedra. In compound 4, a 2D [Ag7I11]4− slab is formed by alternating interconnection of two different types of [Ag7I12] double chains via corner-sharing with one-dimensional (1D) large channels occupied by [(Me)2-2,2′-bipy]2+ cations. UV-vis diffuse-reflectance measurements reveal that the title compounds possess semiconducting behaviors with smaller band gaps of 1.85, 1.78, 1.89 and 2.03 eV, respectively. Sample 4 shows highly efficient photocatalytic degradation activity over organic pollutants than N-doped TiO2 (P25) under visible light irradiation. Moreover, a possible mechanism for the stable photocatalytic activity is proposed based on band structure calculation. Finally, the luminescence properties and thermal stabilities of the title compounds are also studied.

 Please wait while we load your content...
Please wait while we load your content...