Efficient oxygen evolution on hematite at neutral pH enabled by proton-coupled electron transfer†
Abstract
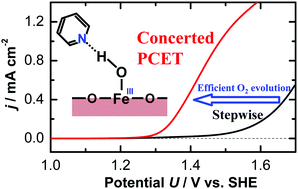
The rate-determining step of the oxygen evolution reaction on hematite electrodes was switched from the sequential electron/proton transfer process to the concerted proton-coupled electron transfer (CPET) process by adding pyridine derivatives to the electrolyte. By inducing the CPET process, the overpotential for oxygen evolution at neutral pH decreased by approximately 250 mV.

 Please wait while we load your content...
Please wait while we load your content...