Abstract
Alkynylzinc reagents were found to undergo coupling with aryl and alkenyl iodides to give arylalkynes and alkenylalkynes without the aid of transition metals. The coupling reaction proceeds through a single electron transfer mechanism, where a substoichiometric amount of a phosphine works as an indispensable activator.
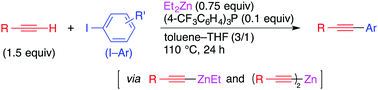


 Please wait while we load your content...
Please wait while we load your content...