Stereoselective synthesis of 2,3,4-highly substituted oxetanes by intramolecular C–C bond forming Michael addition†
Abstract
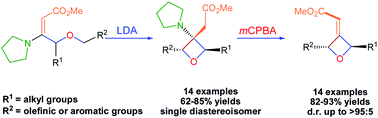
Stereoselective synthesis of 2,3,3,4-tetrasubstituted oxetanes via intramolecular allyl or benzyl anion four-membered ring cyclization of vinylogous urethane derivatives is presented. The resulting products featuring a β-pyrrolidinyl ester at C3 were readily transformed into the corresponding 3-α,β-unsaturated ester substituted oxetanes by Cope elimination reactions.


 Please wait while we load your content...
Please wait while we load your content...