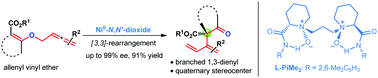
Enantioselective construction of branched 1,3-dienyl substituted quaternary carbon stereocenters by asymmetric allenyl Claisen rearrangement†
Abstract
The availability of enantiomerically enriched 1,3-dienyl substituted quaternary stereocenters is highly valuable for the synthesis of complex natural compounds. Despite great advances in the area of construction of alkenyl-substituted types, a general, practical catalytic system that works for enantioselective formation of 1,3-diene derivatives still remains to be developed. Herein, we disclose the first asymmetric Claisen rearrangement of allenyl vinyl ethers to access optically active β-ketoesters, containing branched 1,3-butadienyl substituted stereocenters. A variety of substrates bearing a range of useful functional groups were well tolerated, thus affording the corresponding products with excellent enantioselectivities (up to 99% ee) and high yields (up to 91%).

 Please wait while we load your content...
Please wait while we load your content...