Cu-Catalyzed hydrophosphorylative ring opening of propargyl epoxides: highly selective access to 4-phosphoryl 2,3-allenols†
Abstract
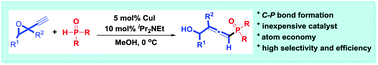
A novel CuI-catalyzed cross-coupling of propargyl epoxides with P(O)H compounds is disclosed. The reaction proceeded efficiently under mild conditions to give 4-phosphoryl 2,3-allenols in good to high yields with excellent selectivity. The utility of the products was demonstrated and a plausible mechanism was also proposed.

 Please wait while we load your content...
Please wait while we load your content...