N-Heterocyclic carbene phosphaketene adducts as precursors to carbene–phosphinidene adducts and a rearranged π-system†
Abstract
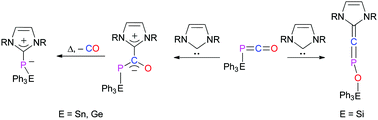
The nucleophilic attack of NHCs on the electron deficient carbon of tetryl substituted phosphaketenes Ph3E–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (E = Sn–Si), leads quantitatively to the formation of NHC–phosphaketene adducts. With E = Sn or Ge, these zwitterionic adducts decompose upon thermolysis under the release of carbon monoxide to give zwitterionic NHC–phosphinidene adducts. With E = Si an OCP to CPO rearrangement occurs which leads to the formation of a linear π-conjugated molecule, NHC
O (E = Sn–Si), leads quantitatively to the formation of NHC–phosphaketene adducts. With E = Sn or Ge, these zwitterionic adducts decompose upon thermolysis under the release of carbon monoxide to give zwitterionic NHC–phosphinidene adducts. With E = Si an OCP to CPO rearrangement occurs which leads to the formation of a linear π-conjugated molecule, NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–O–SiPh3.
P–O–SiPh3.


 Please wait while we load your content...
Please wait while we load your content...