Pd-Catalyzed sequential β-C(sp3)–H arylation and intramolecular amination of δ-C(sp2)–H bonds for synthesis of quinolinones via an N,O-bidentate directing group†
Abstract
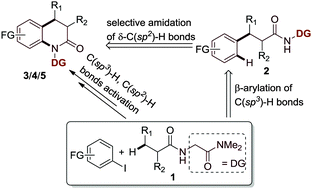
The pharmacological importance of 2-quinolinone derivatives is well known. Herein, we developed an effective protocol for the synthesis of 2-quinolinone derivatives by palladium-catalyzed sequential β-C(sp3)–H arylation and selective intramolecular C(sp2)–H/N–H amination starting with aryl iodides and carboxylic acids. A novel directing group, glycine dimethylamide, was used in the synthesis. We synthesized various quinolinone derivatives, including 5-substituted quinolinones, which are difficult to obtain using the traditional pathway. The directing group could be easily removed and could be readily transformed into other useful functional groups.

 Please wait while we load your content...
Please wait while we load your content...