Lewis acid–base interactions between platinum(ii) diaryl complexes and bis(perfluorophenyl)zinc: strongly accelerated reductive elimination induced by a Z-type ligand†
Abstract
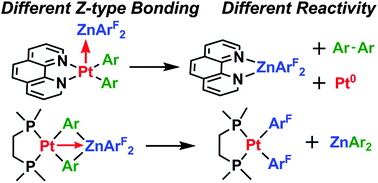
Z-type interactions between bis(perfluorophenyl)zinc and platinum(II) diaryl complexes supported by 1,10-phenanthroline (phen), 2,2′-bipyridine (bpy), and bis(dimethylphosphino)ethane (dmpe) ligands are reported. In the solid state, the nature of the Pt–Zn interaction depends on the bidentate ligand; the phen-supported complex exhibits an unsupported Pt–Zn bond, while the dmpe derivative features additional bridging aryl interactions. A strongly accelerated rate of reductive elimination is observed for phen- and bpy-supported complexes, while aryl exchange between Pt and Zn is observed for the dmpe complex.

 Please wait while we load your content...
Please wait while we load your content...