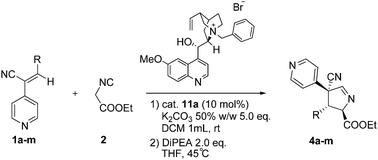
Catalytic asymmetric conjugate addition of isocyanoacetate to (Z)-3-substituted-2-(4-pyridyl)-acrylonitrile, a reactive class of Michael acceptor†
Abstract
(Z)-3-Substituted-2-(4-pyridyl)-acrylonitriles, a reactive class of Michael acceptors obtained exclusively as a single (Z) isomer, reacted with un-substituted isocyanoacetate esters mediated by phase-transfer catalysis to give, after base promoted cyclisation, functionalized imines in up to 94% ee and as a single diastereoisomer.

 Please wait while we load your content...
Please wait while we load your content...