Chemo- and regioselective reductive transposition of allylic alcohol derivatives via iridium or rhodium catalysis†
Abstract
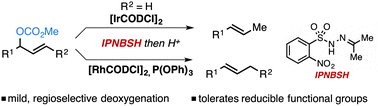
We report highly chemo- and regioselective reductive transpositions of methyl carbonates to furnish olefin products with complementary regioselectivity to that of established Pd-catalysis. These Rh- and Ir-catalysed transformations proceed under mild conditions and enable selective deoxygenation in the presence of functional groups that are susceptible to reduction by metal hydrides.


 Please wait while we load your content...
Please wait while we load your content...