Elucidating the mechanism of peptide interaction with membranes using the intrinsic fluorescence of tryptophan: perpendicular penetration of cecropin B-like peptides into Pseudomonas aeruginosa
Abstract
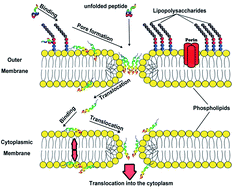
The importance of small molecular weight antimicrobial peptides as novel therapeutic agents stems from their ability to act against bacteria, viruses, and fungi. As part of the innate immune system, they are also capable of killing cancerous cells. Herein, we study the interaction between a synthetic cecropin B peptide and a target Pseudomonas aeruginosa (PA) membrane using steady-state and time-resolved fluorescence measurements in order to elucidate the mechanism of membrane rupture. The importance of synthetic cecropin B as a therapeutic peptide stems from its effect against a wide range of bacteria which is indistinguishable from that of naturally occuring cecropins. Fluorescence of cecropin B results from the sole tryptophan residue in the peptide. In order to understand the mechansim of peptide–membrane binding, we modified the original peptide (cecropin B1: KWKVFKKIEKMGRNIRNGIV) by attaching a terminal tryptophan residue (cecropin B2: KWKVFKKIEKMGRNIRNGIVW). Both peptides show a large inhibition effect against a wide range of bacteria, compared to naturally occurring peptides. The fluorescence results show an enhancement in the peak intensity of cecropin B1 upon mixing with the membrane, accompanied by a blue shift. For cecropin B2, a blue shift was observed upon mixing with the PA membrane, but no enhancement in intensity was observed. The results indicate perpendicular penetration of cecropins B1 and B2 from the Lys side where the Trp residue of cecropin B1 is immersed in the PA membrane. Partial quenching of the Trp fluorescence by acrylamide was observed and the values of the Stern–Volmer constants (Ksv) indicate that the Trp molecule penetrates into the membrane, but resides close to the interface region. Two fluorescence lifetimes were measured for the cecropin B1–PA complex which are for two rotamers of Trp. The results point to a degree of flexibility of the local environment around the Trp molecule. A mechanism of membrane disruption is proposed in which the cecropin peptide creates cracks through the negatively charged outer membrane of PA.

 Please wait while we load your content...
Please wait while we load your content...