Li-ion conductivity in Li9S3N†
Abstract
Li9S3N (LSN) is investigated as a new lithium ion conductor and barrier coating between an electrolyte and Li metal anode in all solid state lithium ion batteries. LSN is an intriguing material since it has a 3-dimensional conduction channel, high lithium content, and is expected to be stable against lithium metal. The conductivity of LSN is measured with impedance spectroscopy as 8.3 × 10−7 S cm−1 at room temperature with an activation energy of 0.52 eV. Cyclic voltammetry (CV) scans showed reversible Li plating and striping. First principles calculations of stability, nudged elastic band (NEB) calculations, and ab initio molecular dynamics (AIMD) simulations support these experimental results. Substitution as a means to enhance conductivity is also investigated. First-principles calculations predict that divalent cation substituents displace a lithium from a tetrahedral site along the migration pathway, and reduce the migration energy for the lithium ions in the vicinity of the substituent. A percolating path with low migration energies (∼0.3 eV) can be formed throughout the crystal structure at a concentration of Li8.5M0.25S3N (M = Ca2+, Zn2+, or Mg2+), resulting in predicted conductivities as high as σ300 K = 2.3 mS cm−1 at this concentration. However, the enhanced conductivity comes at the expense of relatively large substitution energy. Halide substitution, such as Cl on a S site (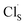 in Kröger–Vink notation), has a relatively low energy cost, but only provides a modest improvement in conductivity.
in Kröger–Vink notation), has a relatively low energy cost, but only provides a modest improvement in conductivity.



 Please wait while we load your content...
Please wait while we load your content...