Ammonia-storage in lithium intercalated fullerides†
Abstract
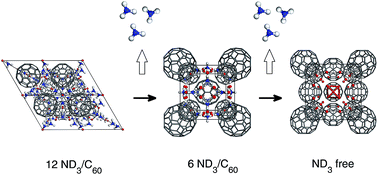
Ammonia has been proposed as an indirect hydrogen carrier, as solid-state ammonia-storage could be easier than directly absorbing hydrogen in materials. Here we investigate the structural evolution of hyper-ammoniated lithium fullerides (ND3)yLi6C60 during ammonia desorption, using in-situ high intensity neutron powder diffraction. In (ND3)yLi6C60, ammonia molecules are stored in their neutral state inside the inter-fullerene interstices and are coordinated to the intercalated Li ions, forming Li–ND3 clusters. Li6C60 is found to absorb up to 36.8 wt% ND3, which corresponds to approximately 14 ammonia molecules per C60. The ammonia release, studied either in-situ or ex-situ by means of manometric analyses and differential scanning calorimetry, takes place in two main steps, at 350–410 K and 500–540 K, respectively. This corresponds to two clear 1st order structural phase transitions and the absorption process is partially reversible. These findings suggest that the system could be a good candidate for ammonia-storage applications.

 Please wait while we load your content...
Please wait while we load your content...