A porous Co(OH)2 material derived from a MOF template and its superior energy storage performance for supercapacitors†
Abstract
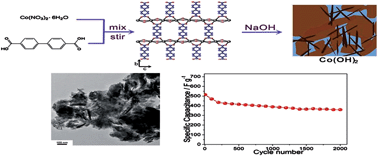
A novel method was explored to synthesize Co(OH)2 using a Co-based metal–organic framework (MOF) as a template via a solid–solid conversion process in alkali solution. The MOF-derived Co(OH)2 was proved to have a loose and mesoporous structure, which can provide good electrolyte penetration, shorten the ion transfer distance, and increase the contact area of the MOF-derived Co(OH)2 and electrolyte. As a result, the as-made Co(OH)2 exhibits superior electrochemical properties with high specific capacitance (604.5 F g−1 at a current density of 0.1 A g−1), excellent rate capability, and good cycling performance. The facile and eco-friendly nature of the strategy give it potential to be extended to other metal hydroxides with excellent electrochemical performances.

 Please wait while we load your content...
Please wait while we load your content...