Encapsulating Ca2Ge7O16 nanowires within graphene sheets as anode materials for lithium-ion batteries†
Abstract
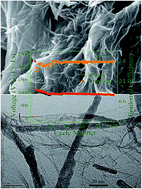
Single-crystal Ca2Ge7O16 nanowires (∼10 nm in diameter) have been encapsulated within graphene sheets successfully to form a nanocomposite with three-dimensional hierarchical nanostructure by a facile hydrothermal method on a large scale. The as-synthesized Ca2Ge7O16 nanowire/graphene sheet nanocomposite electrode exhibits high specific capacity (ca. 950 mA h g−1 at a current density of 100 mA g−1), good cyclability, and excellent rate capability (ca. 400 mA h g−1 at a current density of 3200 mA g−1). Such superior lithium storage performance, on the one hand, could be assigned to the graphene sheets which act as conductive substrates and elastic networks to disperse and encapsulate the Ca2Ge7O16 nanowires, thereby effectively relieving the volume changes and aggregation of the nanoparticles during Li+ insertion/extraction. And on the other hand, the synergistic effect of the unique nanostructured hybrid, in which the Ca2Ge7O16 nanowires are well-stabilized by graphene sheets with high conductivity and flexibility, is beneficial to enlarge the specific surface area, deliver enough sites to allow dispersion of the Ge nanoparticles, and provide void space to buffer the volume change during discharge/charge cycles. This work provides an effective strategy for the fabrication of functionalized ternary-oxide-based composites as high-performance electrode materials that exhibit good conductivity and eliminate severe electrode pulverization.

 Please wait while we load your content...
Please wait while we load your content...