Energetics of lanthanide cobalt perovskites: LnCoO3−δ (Ln = La, Nd, Sm, Gd)
Abstract
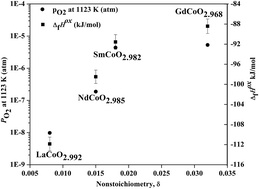
Lanthanide cobalt perovskites LnCoO3−δ (Ln = La, Nd, Sm, and Gd) are important materials for electroceramics, catalysts, and electrodes in solid oxide fuel cells. Formation enthalpies of LnCoO3−δ compounds were measured using high temperature oxide melt solution calorimetry. The formation enthalpies of LaCoO2.992, NdCoO2.985, SmCoO2.982 and GdCoO2.968 from constituent binary oxides (Ln2O3, CoO) and O2 gas are −111.87 ± 1.36, −98.49 ± 1.33, −91.56 ± 1.46 and −88.16 ± 1.45 kJ mol−1, respectively. Thus these perovskites become energetically less stable with decrease in ionic radius of the lanthanide (from La to Gd), which corresponds to a decreasing tolerance factor and increasing oxygen deficiency. The thermodynamic stability of LaCoO2.992, NdCoO2.985, SmCoO2.982 and GdCoO2.968 was also assessed considering their oxygen partial pressures for decomposition, with good agreement between thermochemical and equilibrium data.

 Please wait while we load your content...
Please wait while we load your content...