Insight into the electrochemical activation of carbon-based cathodes for hydrogen evolution reaction†
Abstract
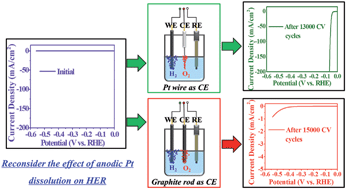
Recently, carbon nanomaterials with outstanding electrocatalytic performance for the hydrogen evolution reaction (HER) after electrochemical activation have been reported; however, the exact activation mechanism is still under extensive debate. In this study, to better understand the activation, graphite rods and carbon nanohorns, two typical carbon materials in different scales, were electrochemically activated and their catalytic performances in HER were systematically studied, which showed that the HER performance was greatly affected by the counter electrode employed for the activation. An efficient activation was achieved when a platinum wire was used as the counter electrode; simultaneously, Pt transfer from the anode to the cathode was also observed. These results suggest that the improved HER performance was mainly caused by the Pt transfer, rather than the activation of the carbon materials themselves. More importantly, our study implied that the Pt dissolution, although widely ignored, should be taken into consideration during electrochemical tests when Pt metal is utilized as the counter electrode.

 Please wait while we load your content...
Please wait while we load your content...