Carbon nanotubes decorated with nickel phosphide nanoparticles as efficient nanohybrid electrocatalysts for the hydrogen evolution reaction†
Abstract
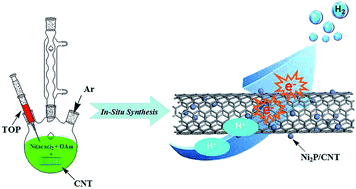
Designing efficient, stable and inexpensive electrocatalysts to replace Pt-based catalysts for the hydrogen evolution reaction (HER) is highly desired in renewable energy research. In this study, we report the synthesis of nickel phosphide nanoparticles decorated on multiwalled carbon nanotubes (Ni2P/CNT) by in situ thermal decomposition of nickel acetylacetonate as a nickel source and trioctylphosphine as a phosphorus source in oleylamine solution of CNT. As a novel HER electrocatalyst, the Ni2P/CNT nanohybrid exhibits excellent electrocatalytic activity in 0.5 M H2SO4 with a low onset overpotential (88 mV), a small Tafel slope (53 mV dec−1), a high exchange current density (0.0537 mA cm−2) and good stability. It only needs overpotentials of 98 and 124 mV to attain current densities of 2 and 10 mA cm−2, respectively. In addition, the Ni2P/CNT nanohybrid shows nearly 100% Faradaic efficiency in acid solutions. This work successfully demonstrates that the introduction of Ni2P NPs into CNT for enhanced electrocatalytic properties is feasible, and that this may open up a potential way for designing more efficient Ni2P-based catalysts for the HER.

 Please wait while we load your content...
Please wait while we load your content...