Salt-induced counterion condensation and related phenomena in sodium carboxymethylcellulose–sodium halide–methanol–water quaternary systems
Abstract
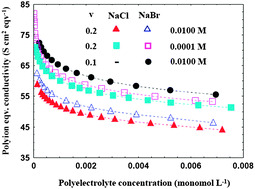
Polyion–counterion interactions in sodium carboxymethylcellulose (NaCMC) in methanol–water media have been investigated conductometrically with reference to their variations with polyelectrolyte concentration, relative permittivity and the type and concentration of added electrolytes. The specific conductance data in polyelectrolyte–salt solutions were analyzed using an equation recently developed by us following the scaling description for the configuration of a polyion chain according to Dobrynin et al. Excellent quantitative agreement between the experimental results and those obtained with the new equation developed was observed. The results demonstrate that approximately 43–59% of the counterions remain free and that there has been a suppression of counterion dissociation in the presence of a salt in any given mixed solvent medium, the extent of which increases with increasing salt concentration. NaCl was found to be slightly more efficient than NaBr in suppressing the counterion-condensation in NaCMC–methanol–water systems. An increase in the amount of methanol in the media causes a reduction in the fraction of free counterions. The results further demonstrate that the monomer units experience more frictional resistance as the methanol content of the mixture increases or as the concentration of the added electrolytes increases. The results were discussed in terms of various interactions prevailing in these systems.

 Please wait while we load your content...
Please wait while we load your content...