Metal influence on the iso- and hetero-selectivity of complexes of bipyrrolidine derived salan ligands for the polymerisation of rac-lactide†
Abstract
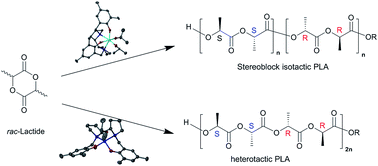
In this paper we have prepared a series of Ti(IV), Hf(IV) and Al(III) complexes based on bipyrrolidine salan pro-ligands. The Hf(IV) complexes have all been characterised in the solid-state, the chiral ligands coordinate to Hf(IV) in an α-cis manner whereas the meso ligand coordinates in a β-cis geometry. The Hf(IV) complexes are all active for the ROP of rac-lactide in the melt, with the fluxional meso complex affording a strong isotactic bias Pm = 0.84. As expected Hf(3)(OiPr)2 polymerised L-LA faster than rac-LA (kapp = 5.9 × 10−3 min−1vs. 3.8 × 10−3 min−1). For Ti(IV) complexes atactic PLA was formed. The salan pro-ligands have also been complexed to Al(III), and the novel Al–Me and Al-OiPr complexes were characterised in the solid and solution state. Al(1)(OiPr) was fluxional on the NMR timescale, whereas Al(3)(OiPr) was locked in solution with no exchange. Interestingly, the Al(III) complexes of 3H2 produce PLA with a very strong heterotactic bias Pr upto 0.87, whereas atactic PLA is produced with 1H2. For Al(3)(OiPr) a linear relationship is observed with Mn and conversion. Experiments with the addition of an equivalent of rac-LA to the selective initiators have also been performed and are discussed.


 Please wait while we load your content...
Please wait while we load your content...