Stereoselective synthesis of macrocyclic peptides via a dual olefin metathesis and ethenolysis approach†
Abstract
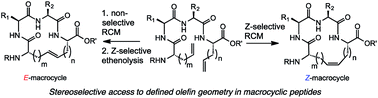
Macrocyclic compounds occupy an important chemical space between small molecules and biologics and are prevalent in many natural products and pharmaceuticals. The growing interest in macrocycles has been fueled, in part, by the design of novel synthetic methods to these compounds. One appealing strategy is ring-closing metathesis (RCM) that seeks to construct macrocycles from acyclic diene precursors using defined transition-metal alkylidene catalysts. Despite its broad utility, RCM generally gives rise to a mixture of E- and Z-olefin isomers that can hinder efforts for the large-scale production and isolation of such complex molecules. To address this issue, we aimed to develop methods that can selectively enrich macrocycles in E- or Z-olefin isomers using an RCM/ethenolysis strategy. The utility of this methodology was demonstrated in the stereoselective formation of macrocyclic peptides, a class of compounds that have gained prominence as therapeutics in drug discovery. Herein, we report an assessment of various factors that promote catalyst-directed RCM and ethenolysis on a variety of peptide substrates by varying the olefin type, peptide sequence, and placement of the olefin in macrocycle formation. These methods allow for control over olefin geometry in peptides, facilitating their isolation and characterization. The studies outlined in this report seek to expand the scope of stereoselective olefin metathesis in general RCM.

 Please wait while we load your content...
Please wait while we load your content...