Low temperature ionic conductor: ionic liquid incorporated within a metal–organic framework†
Abstract
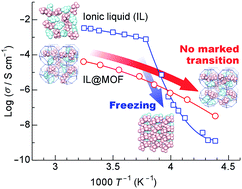
Ionic liquids (ILs) show promise as safe electrolytes for electrochemical devices. However, the conductivity of ILs decreases markedly at low temperatures because of strong interactions arising between the component ions. Metal–organic frameworks (MOFs) are appropriate microporous host materials that can control the dynamics of ILs via the nanosizing of ILs and tunable interactions of MOFs with the guest ILs. Here, for the first time, we report on the ionic conductivity of an IL incorporated within a MOF. The system studied consisted of EMI-TFSA (1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide) and ZIF-8 (Zn(MeIM)2, H(MeIM) = 2-methylimidazole) as the IL and the MOF, respectively. While the ionic conductivity of bulk EMI-TFSA showed a sharp decrease arising from freezing, the EMI-TFSA@ZIF-8 showed no marked decrease because there was no phase transition. The ionic conductivity of EMI-TFSA@ZIF-8 was higher than that of bulk EMI-TFSA below 250 K. This result points towards a novel method by which to design electrolytes for electrochemical devices such as batteries that can operate at low temperatures.

 Please wait while we load your content...
Please wait while we load your content...