Cu-catalyzed transannulation reaction of pyridotriazoles with terminal alkynes under aerobic conditions: efficient synthesis of indolizines†
Abstract
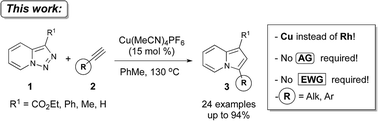
A Cu(I)-catalyzed denitrogenative transannulation reaction of pyridotriazoles with terminal alkynes en route to indolizines was developed. Compared to the previously reported Rh-catalyzed transannulation reaction, this Cu-catalyzed method features aerobic conditions and a much broader scope of pyridotriazoles and alkynes.

 Please wait while we load your content...
Please wait while we load your content...