A combination of polyunsaturated fatty acid, nonribosomal peptide and polyketide biosynthetic machinery is used to assemble the zeamine antibiotics†
Abstract
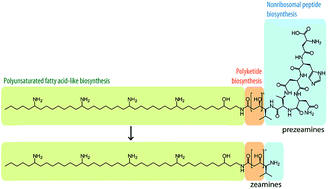
The zeamines are a unique group of antibiotics produced by Serratia plymuthica RVH1 that contain variable hybrid peptide–polyketide moieties connected to a common pentaamino-hydroxyalkyl chain. They exhibit potent activity against a broad spectrum of Gram-positive and Gram-negative bacteria. Here we report a combination of targeted gene deletions, high resolution LC-MS(/MS) analyses, in vitro biochemical assays and feeding studies that define the functions of several key zeamine biosynthetic enzymes. The pentaamino-hydroxyalkyl chain is assembled by an iterative multienzyme complex (Zmn10–13) that bears a close resemblance to polyunsaturated fatty acid synthases. Zmn14 was shown to function as an NADH-dependent thioester reductase and is proposed to release a tetraamino-hydroxyalkyl thioester from the acyl carrier protein domain of Zmn10 as an aldehyde. Despite the intrinsic ability of Zmn14 to catalyze further reduction of aldehydes to alcohols, the initially-formed aldehyde intermediate is proposed to undergo preferential transamination to produce zeamine II. In a parallel pathway, hexapeptide-monoketide and hexapeptide-diketide thioesters are generated by a hybrid nonribosomal peptide synthetase-polyketide synthase multienzyme complex (Zmn16–18) and subsequently conjugated to zeamine II by a stand-alone condensing enzyme (Zmn19). Structures for the resulting prezeamines were elucidated using a combination of high resolution LC-MS/MS and 1- and 2-D NMR spectroscopic analyses. The prezeamines are hypothesized to be precursors of the previously-identified zeamines, which are generated by the action of Zmn22, an acylpeptide hydrolase that specifically cleaves the N-terminal pentapeptide of the prezeamines in a post-assembly processing step. Thus, the zeamine antibiotics are assembled by a unique combination of nonribosomal peptide synthetase, type I modular polyketide synthase and polyunsaturated fatty acid synthase-like biosynthetic machinery.

 Please wait while we load your content...
Please wait while we load your content...